Advancing Concussion
Therapy, On and Off the Field
TecTraum Inc. has designed and developed the world’s first-ever medical device treatment for concussions, potentially reducing the signs and symptoms of concussion. Designated by the U.S. Food and Drug Administration as a Breakthrough Device, pro2cool® offers the potential for clinically significant improvements for patients who have suffered a mild traumatic brain injury (mTBI, or concussion) in a low-risk, easy-to-use, point-of-care delivery system. Currently, the pro2cool® system is an investigational use only device and has not yet received market authorization from the FDA.
Functional
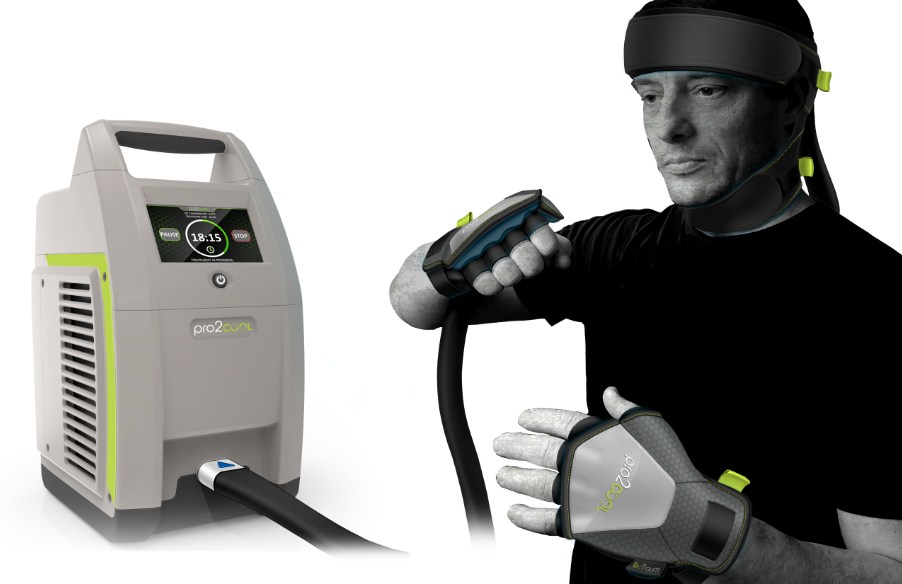
pro2cool® is a small, easy-to-use and portable system that can accompany any athletic team, yet precise enough for any emergency room, doctor’s office, and school facility. The goal of creating this product was to develop a safe, effective, efficient, and easy-to-use medical device that could be deployed in almost any setting.
Clinical Efficacy
The pro2cool® device leverages hypothermic therapy (“cold therapy”), which has been shown to have clinical efficacy in a variety of cardiovascular injuries, including cardiac arrest and myocardial infarction (heart attack). Researchers investigating the pro2cool™ device have observed significant improvements in clinical outcomes through the cooling of the brain within eight days of the concussion.
Non-Invasive
pro2cool® is non-invasive and simple to use, delivering precise amounts of cooling therapy. With few moving parts, the device can be assembled in minutes and put to work immediately upon on the concussed patient, or within eight days of the injury.
My Concussion Story
Mitchell Schwartz
“Going through the process of designing and developing the pro2cool® system and learning more about cooling therapy as a neuro-protectant gives us the confidence to say, not only is it possible hypothermic cooling of the brain works for treating concussions, but it is rather probable.”
— Sam Williams and Justin Fargas, former NFL football players
pro2cool® is considered a Class 2 medical device that first received FDA Breakthrough Device designation in June 2021. A large multi-site clinical trial for pro2cool® was completed in April 2022, with planned submission of data to the FDA for consideration of market authorization in Q4 2023. Currently, the pro2cool® system is an investigational use only device and has not yet received market authorization from the FDA.

